» Tags » Cleaning Validation
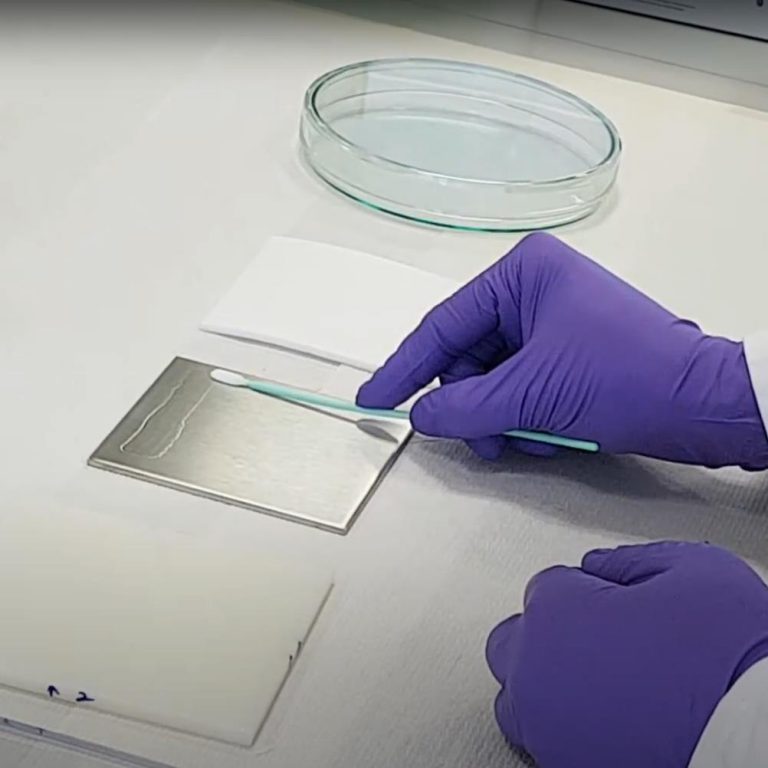
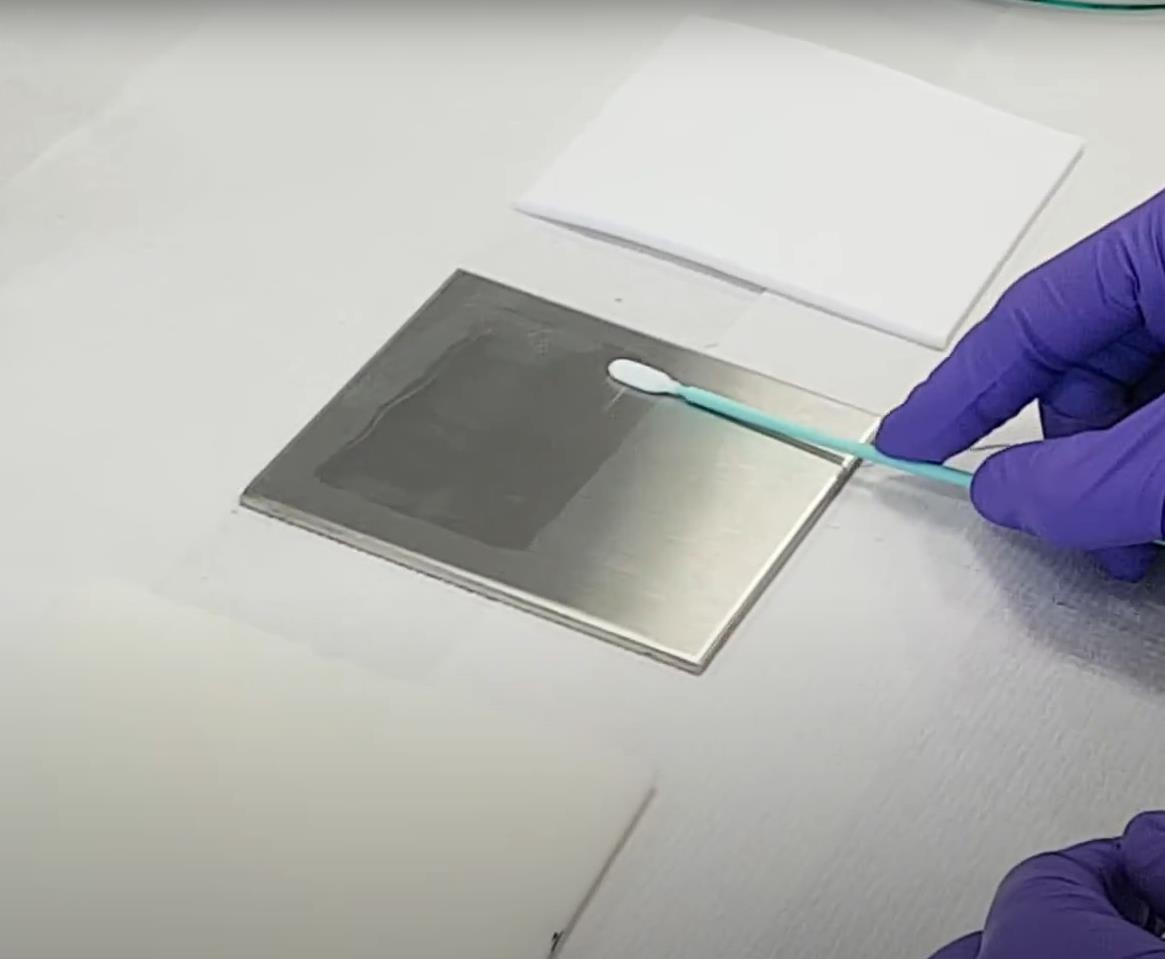
Procedure for Swab Sampling for Validation of Test Surface to Evaluate Cleaning Efficacy
Swab sampling procedure: 1. Pipette out 5 ml of sampling solvent in the transport container. 2. Remove a swab from its protective bag using a clean latex hand glove. 3. Avoid touching the swab head to prevent its contamination. 4. Transfer the swab in transport container (test tube) containing 5 ml of sampling solvent and allow the swab to soak …
The Four Most Important Criteria to Choose a Swab for Cleaning Validation
In pharmaceutical manufacturing, the product must not be contaminated by previous product as well as it should not be contaminated with the cleaning agent used for equipment cleaning. To verify the cleaning process it is validated by taking the sample from various parts of cleaned equipment that is to be used in the manufacturing process and these samples are analyzed …
Direitos autorais © 2023. Medico Technology Co., Ltda