Application of Cleanroom Swabs in the Bio-medical Field
Cleanroom swabs are indispensable tools in modern bio-medical workflows where precision, sterility, and traceability are non-negotiable. From sample collection to device manufacturing and contamination control, the right swab material and design can significantly impact outcomes, yield, and compliance. What Are Cleanroom Swabs? Cleanroom swabs are precision-manufactured applicators assembled and packaged in controlled environments to minimize particulate, ionic, and microbial contamination. …
TOC Cleaning Validation Kits Instructions: Step-by-Step Guide
Total Organic Carbon (TOC) cleaning validation is a critical process in pharmaceutical and biotechnology industries. Proper use of a TOC cleaning validation kits ensures your facility meets regulatory standards and maintains product quality. This guide provides clear, step-by-step instructions for using your TOC cleaning validation kits effectively. Preparation Before beginning, always consult your written Standard Operating Procedures (SOPs) for specific …
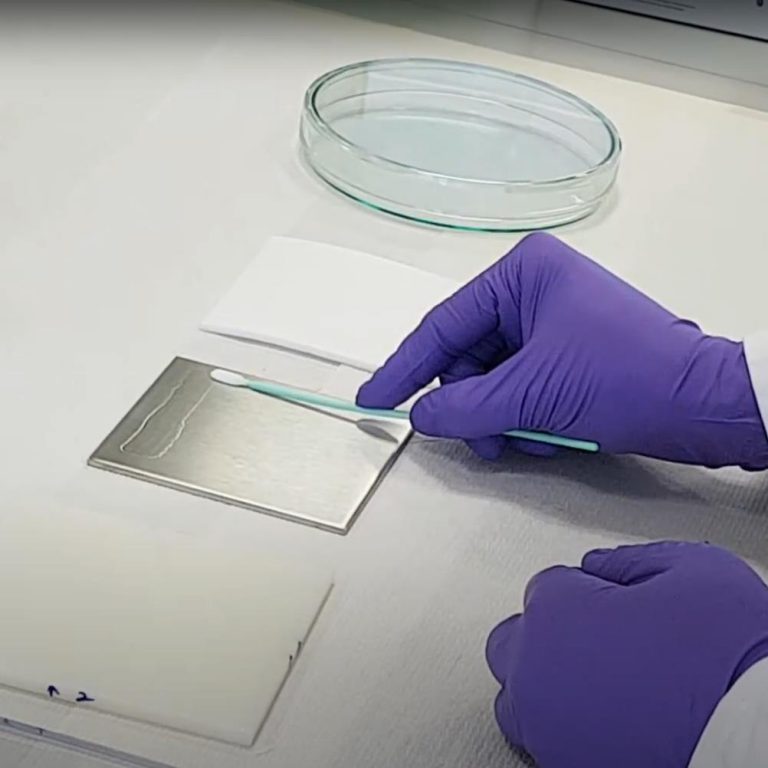
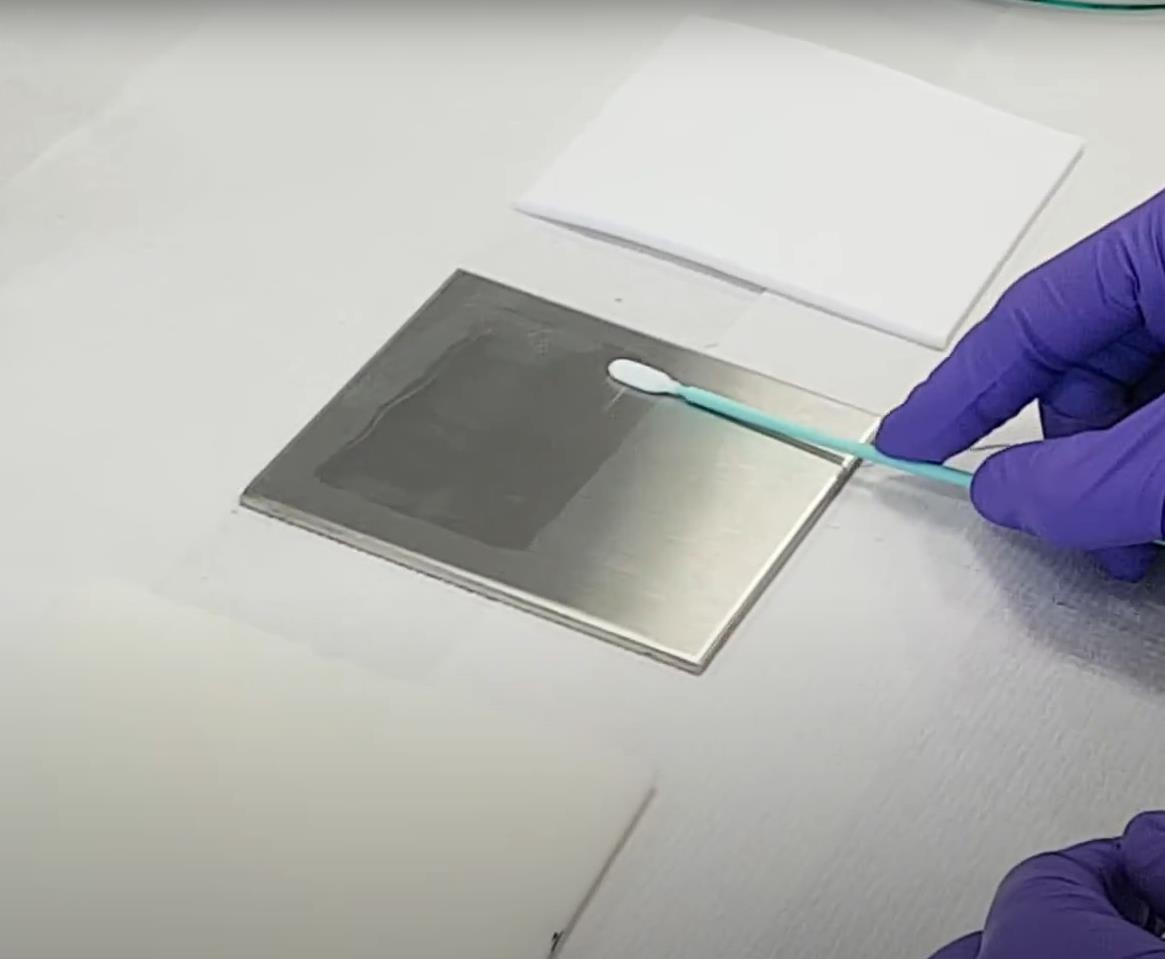
Procedure for Swab Sampling for Validation of Test Surface to Evaluate Cleaning Efficacy
Swab sampling procedure: 1. Pipette out 5 ml of sampling solvent in the transport container. 2. Remove a swab from its protective bag using a clean latex hand glove. 3. Avoid touching the swab head to prevent its contamination. 4. Transfer the swab in transport container (test tube) containing 5 ml of sampling solvent and allow the swab to soak …
The Four Most Important Criteria to Choose a Swab for Cleaning Validation
In pharmaceutical manufacturing, the product must not be contaminated by previous product as well as it should not be contaminated with the cleaning agent used for equipment cleaning. To verify the cleaning process it is validated by taking the sample from various parts of cleaned equipment that is to be used in the manufacturing process and these samples are analyzed …